A team of UCLA bioengineers has demonstrated that its technology may go a long way toward overcoming the challenges of treatment for acute lymphoblastic leukemia, among the most common types of cancer in children, and has the potential to help doctors personalize drug doses.
The five-year survival rate for individuals with pediatric acute lymphoblastic leukemia is about 85 percent, however those who experience a recurrence generally have a poor prognosis and a bone marrow transplant is their only option for a permanent cure.
Conventional treatment for this leukemia includes a combination of drugs, which come with short- and long-term side effects. Two of these drugs, 6-mercaptopurine and methotrexate, can cause liver disease and other life-threatening infections. During the maintenance phase of treatment, which aims to keep individuals in remission, dosing for these two drugs is frequently adjusted through a system of trial and error, which is not always accurate.
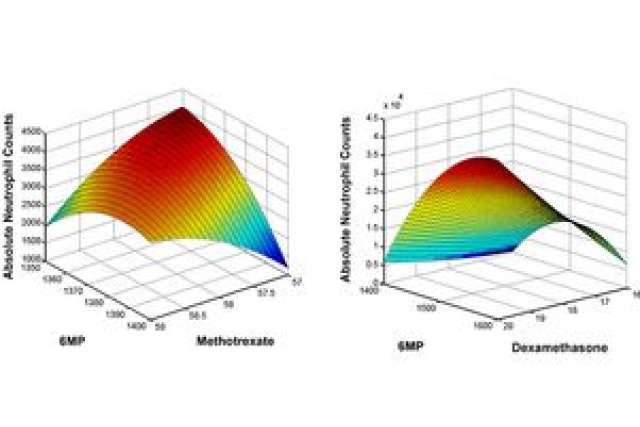
The UCLA team from the Schools of Dentistry, Medicine and Engineering showed that its digital health technology platform, called Phenotypic Personalized Medicine, or PPM, can surmount the treatment challenges for this leukemia. The technology platform is based on the team’s that a patient’s physical response to drug treatment, such as tumor size or bacterial and viral levels in the blood, could be visually represented in the shape of a parabola, or U-shaped line. The graphs plot the drug dose along the horizontal axis and the patient’s response to treatment on the vertical axis.
The technology has the ability to accurately identify a person’s optimal drug and dose combinations throughout an entire course of treatment. In addition, the technology platform does not require any complex and expensive analysis of a patient’s genetic information or the biological basis of the disease, greatly accelerating the ability to optimize and personalize care.
The team’s findings appear in the peer-reviewed journal SLAS Technology, which features innovations in technology for drug development and diagnostics.
“Phenotypic Personalized Medicine is like turbocharged artificial intelligence. It personalizes combination therapy to optimize efficacy and safety,” said Dean Ho, co-corresponding author of the study and professor of oral biology and medicine. “The ability for our technology to continuously pinpoint the proper dosages of multiple drugs from such a large pool of possible combinations overcomes a challenge that is substantially more difficult than finding a needle in a haystack,” added Ho, who is also the co-director of the Weintraub Center for Reconstructive Biotechnology at the UCLA School of Dentistry.
In this study, patient records were obtained on the dosing of 6-mercaptopurine and methotrexate, as well as on the corresponding absolute neutrophil count, or levels of a subset of white blood cells called neutrophils that are vital for staving off potentially life-threatening infections. These records showed multiple instances where conventional chemotherapy doses caused deviations from acceptable neutrophil levels. Using the personalized medicine technology, individualized three-dimensional maps were generated to determine the optimal 6-mercaptopurine/methotrexate drug ratios.
The team members found that their technology-suggested drug dosages were as much as 40 percent lower compared to clinical chemotherapy dosages, while still maintaining target neutrophil levels. The parabolas showed that markedly different dosages of each drug were required to maintain normal white blood cell counts for each patient. Their results demonstrated a clear need to personalize acute lymphoblastic leukemia treatment, and will serve as a foundation for a pending clinical trial to optimize multi-drug chemotherapy.
“PPM has the ability to personalize combination therapy for a wide spectrum of diseases, making it a broadly applicable technology,” said Chih-Ming Ho, distinguished research professor of mechanical and aerospace engineering and co-corresponding author of the study. “The fact that we don’t need any information pertaining to a disease’s biological process in order to optimize and personalize treatment is a revolutionary advance. We’re at the interface of digital health and cancer treatment.”
Dr. Vivian Chang, co-first author of the study and assistant professor of pediatrics and hematology and oncology, said, “Optimizing combination therapy for [pediatric leukemia] on a patient-specific level would be a game-changer for the way that this cancer, as well as many other cancers, is addressed. Reducing side effects while maintaining or even enhancing efficacy could also improve the long-term treatment outcomes of our patients.” Chang is also co-director of the Pediatric Cancer Predisposition Clinic at UCLA.
The research team is planning to recruit patients for a prospective trial within the next year. The technology is approved for additional infectious disease and oncology studies.
Other authors of the study, all from UCLA, are co-first author and postdoctoral researcher Dong-Keun Lee and graduate student Theodore Kee. Dean Ho, Chih-Ming Ho and Chang are also members of the Jonsson Comprehensive Cancer Center.
This work was supported by the National Cancer Institute, National Science Foundation, V Foundation for Cancer Research, Wallace H. Coulter Foundation, Society for Laboratory Automation and Screening, UCLA Children’s Discovery and Innovation Institute, Today’s and Tomorrow’s Children Fund Award and the endowment fund of the Ben Rich–Lockheed Martin Professorship.