A Comprehensive Cancer Center

One of the Top Cancer Centers in America
The UCLA Health Jonsson Comprehensive Cancer Center (UCLA Health JCCC) attained designation as a "comprehensive cancer center" by the National Cancer Institute (NCI) in 1976, a testament to its sustained excellence in cancer research, education, and outreach into Los Angeles County communities and well beyond. A strong partnership with UCLA Health provides outstanding cancer patient care, prevention, and survivorship.
UCLA Health Jonsson Comprehensive Cancer Center is consistently recognized as a top center in the nation for cancer care by U.S. News & World Report and has a long record of excellence by this and other measures.
At UCLA Health, teams of specialists work together to treat and assist cancer patients and their families. A multidisciplinary approach helps patients and family members confront both the immediate and long-term effects of cancer. As an NCI-designated comprehensive cancer center, the UCLA Health JCCC works to reduce the burden of cancer in our communities and to ensure that all benefit from medical advances that result from cancer research.
The most advanced cancer treatment
The UCLA Health JCCC has an international reputation for developing new cancer therapies, providing the best in experimental and traditional treatments. Center members conduct research on a wide range of cancers and the Center offers patients the opportunity to participate in clinical trials and research. The UCLA Health JCCC has over 500 active cancer clinical trials at any given time that are enrolling patients, and offers many of these trials not only to residents of Los Angeles County but at partner institutions and affiliate sites across the country.
Uncovering breakthroughs in cancer prevention and treatment
With more than 500 physicians and scientists, the UCLA Health JCCC is one of the largest comprehensive cancer centers in the nation. With an international reputation for providing the best in experimental and traditional cancer treatments, UCLA Health JCCC experts also train and educate the next generation of medical researchers. Successful targeted cancer therapies such as trastuzumab, imatinib, enzalutamide, and others were developed from basic studies performed in Center laboratories and brought to clinical trials for UCLA cancer patients.
National Comprehensive Cancer Network
The UCLA Health JCCC is also one of 33 leading cancer centers in the United States participating in the National Comprehensive Cancer Network (NCCN). As a NCCN member, UCLA Health JCCC experts join experts from other Centers in setting the standards for diagnosing and treating patients with cancer, with a particular focus on uncommon, complex, or aggressive forms of the disease.
History & mission
In the late 1960s, a group of scientists, clinicians, and volunteers at UCLA came together to develop a cancer center that would become renowned for excellence in research, education, and patient care. Today, the UCLA Health Jonsson Comprehensive Cancer Center (UCLA Health JCCC) is a global leader in cancer research, education, and clinical care with a mission to accelerate discoveries to prevent and cure cancer.
The UCLA Health JCCC is one of only 57 National Cancer Institute (NCI)-designated comprehensive cancer centers in the nation, attaining the founders’ aspiration with the highest designation possible from the NCI. The NCI recognizes comprehensive cancer centers from around the country that meet rigorous standards for transdisciplinary, state-of-the-art research focused on developing new and better approaches to preventing, diagnosing, and treating cancer for the communities these centers serve. The UCLA Health JCCC has held this
Find Your Care
Call our cancer hotline at for more information on cancer programs offered at UCLA Health.
Why Choose Our Cancer Center?
Leaders in the field
We have more than 500 scientists and physicians who conduct basic and translational cancer research and provide comprehensive cancer care
Cutting-edge vaccines
UCLA neuroscientists developed one of the first personalized vaccines for people with glioblastoma, the deadliest type of brain tumor.
Transforming treatment
Since 2014, research at the UCLA Health Jonsson Comprehensive Cancer Center has led or contributed to 22 approvals from the FDA for new cancer treatments or protocols, a remarkable achievement.
State-of-the-art research
We have over 400 active clinical trials focused on cancer care that are currently enrolling patients at UCLA
Life-changing medicine
UCLA research led to the development of the breast cancer drug Herceptin, developed by world renowned cancer researcher Dr. Dennis Slamon.
Pioneering immunotherapies
In the largest Phase 1 study in the history of oncology, UCLA researchers led by Dr. Antoni Ribas co-developed the first-of-its-kind immunotherapy Keytruda to treat melanoma.
Decades of experience
The UCLA Health Jonsson Comprehensive Cancer Center has held the highest designation possible from the National Cancer Institute (NCI) since 1976.
World-class cancer care
The UCLA Health Jonsson Comprehensive Cancer Center is ranked as one of the nation's “Best Hospitals” in cancer by U.S. News & World Report.
Dr. Dennis Slamon discovered the relationship between the HER-2/neu gene and an aggressive form of breast cancer found in about 30 percent of patients. This discovery led to the development of the new breast cancer drug Herceptin, approved by the FDA on September 25, 1998, making it the first approved treatment that attacks cancer at its genetic roots.

Developed by Dr. Antoni Ribas, Keytruda is the first-of-its-kind immunotherapy approved by the FDA on September 14, 2014 to treat advanced melanoma. Signaling a paradigm shift in the way the deadly skin cancer is treated, the research was conducted at UCLA and 11 other sites across the world as part of the largest phase 1 clinical study in the history of oncology.

Building upon Ribas' research, Dr. Edward Garon led the largest study published to date using immunotherapy to treat lung cancer. It was approval by the FDA on October 2, 2015 for use of Keytruda in advanced non-small cell lung cancer. In October 2014 the FDA granted the drug "breakthrough therapy" status, allowing it to be fast-tracked for approval.
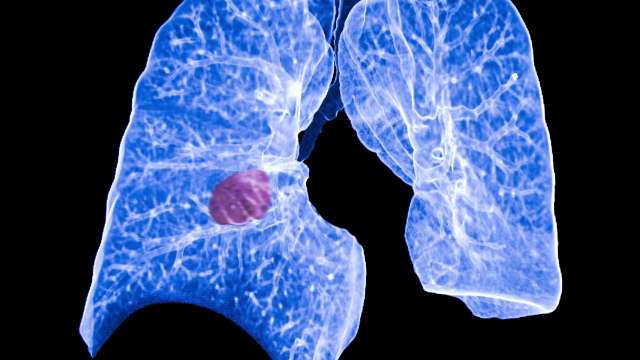
In 2014, Garon was also the principal investigator on a multi-year, international phase 3 clinical trial of Cyramza. Tested on more than 1,200 patients, the drug was found to extend survival significantly in NSCLC and approved by the FDA on December 12, 2014.

Slamon and Dr. Richard Finn developed IBRANCE, a groundbreaking new treatment strategy to arrest tumor growth in women with estrogen-receptor positive advanced breast cancer. It was approved by the FDA on February 3, 2015.
UCLA was one of three sites nationwide to conduct the first human tests on Gleevec, a once-a-day pill for a common form of adult leukemia called chronic myelogenous leukemia. Much of the pioneering work done to link the Bcr-Abl gene and its mutant protein with CML was done at UCLA by Dr. Owen Witte and Dr. Charles Sawyers.
Advancements in Translational Research
Sarcoma
Dr. Fred Eilber developed a limb salvage technique that now serves as a national model. He treats sarcoma patients with chemotherapy before surgery, avoiding limb amputation that would otherwise be required.
Ovarian cancer
Dr. Mark Pegram led the team of Jonsson Cancer Center doctors who were the first to treat ovarian cancer patients with gene therapy using the p53 tumor suppressor gene.
Kidney cancer
Drs. Robert Figlin and Arie Belldegrun developed a new approach to treating metastatic kidney cancer by combining biologic and cellular therapies to extend remission times.
Patient recovery
Dr. Judith Gasson and colleagues purified GM-CSF, which shortened the time it takes for cancer patients to recover their white blood cell counts (from five to two weeks) after bone marrow transplants.
BMT
Dr. John Glaspy and Dr. Mary Territo used megakaryocyte growth and development factor (MGDF) for the first time to speed up a cancer patient´s bone marrow recovery after transplant.
Diet and nutrition
Glaspy demonstrated for the first time that dietary regulation of certain fatty acids can change the composition of human breast tissue in such a way that it may be more resistant to cancer.
Breast cancer
In 1992, UCLA Health opened the first multidisciplinary breast cancer center on the West Coast, the , for women with breast problems or at high risk of developing the disease.
Prostate cancer
Dr. Charles Sawyers developed the first animal model for prostate cancer, and the ability to grow those cells in animals provided researchers with a crucial new research tool.