Radial Sclerosing Lesions
Naomi Walker, MD and Nooshin Najmi, MD
Background:
Radial sclerosing lesions (RSLs) are non-malignant breast lesions characterized by entrapped benign epithelial elements distorted by fibroelastosis and hyalinized stroma (1). Radial sclerosing lesions include both radial scars and complex sclerosing lesions and are often used interchangeably with these terms. Radial scars and complex sclerosing lesions are differentiated by their histopathologic architecture. Radial scars demonstrate a stellate configuration with ducts radiating from a fibroelastic core, while complex sclerosing lesions have a more disorganized architecture and are larger in size measuring >10 mm (2). Proliferative changes and calcifications may be present in both (2). Though RSLs may be identified in isolation, they may also be associated with atypia or high-risk lesions with malignant potential, ultimately affecting the risk of malignancy and management of these lesions (1,2).
Pathogenesis:
The pathogenesis of RSLs remains uncertain. Given the overlap in the histologic features of sclerosing papilloma and CSLs, it is hypothesized that CSLs may be a later stage of a sclerosing papilloma. Radial sclerosing lesions are not found to be associated with prior surgery or injury, parity, or contraceptive use(2).
Clinical Presentation & Epidemiology:
Radial sclerosing lesions are clinically occult and most frequently identified in women aged 30-60 years(3). These lesions are often not palpable or associated with skin retraction or thickening regardless of their size or location in the breast. Radial sclerosing lesions may be found incidentally in breast tissue that is removed for other reasons or may be detected on imaging and targeted for biopsy. The incidence of RSLs ranges from 0.6-3.7% at core needle biopsy and the frequency ranges from 14-28% in several autopsy series (2,3).
Imaging Findings:
Mammography:
Radial sclerosing lesions are often identified incidentally on screening mammograms. There is robust evidence that digital breast tomosynthesis improves the detection and characterization of RSLs compared to digital mammography (4). The radiographic appearance of RSLs may be indistinguishable from that of invasive carcinoma, thus needle biopsy is essential in excluding malignancy.
Radial sclerosing lesions may be multiple and/or bilateral. The most typical appearance is an architectural distortion or the “black star” configuration, which is defined as spiculations radiating from a central point without a central mass (Fig 1a). However, the presence of a radiolucent center is not reliable in differentiating a RSL from malignancy (1). Less frequently, RSLs may appear as a stellate opacity or the “white star,” which is described as a mass with spiked linear extensions (3).
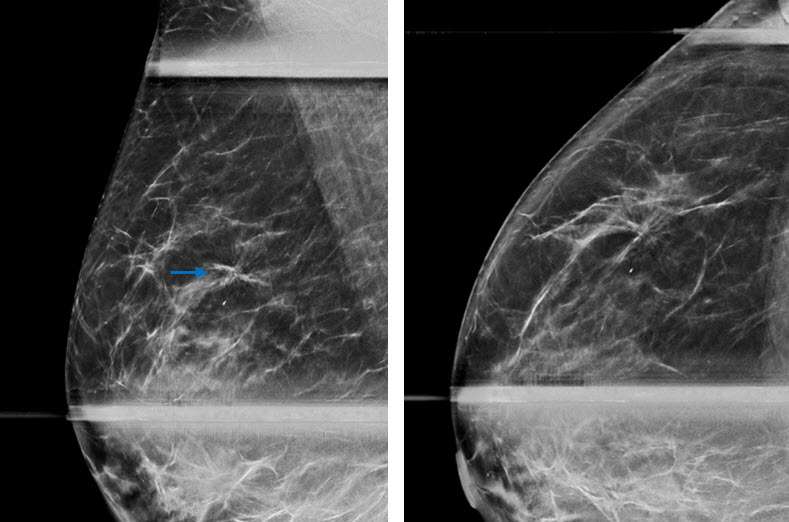
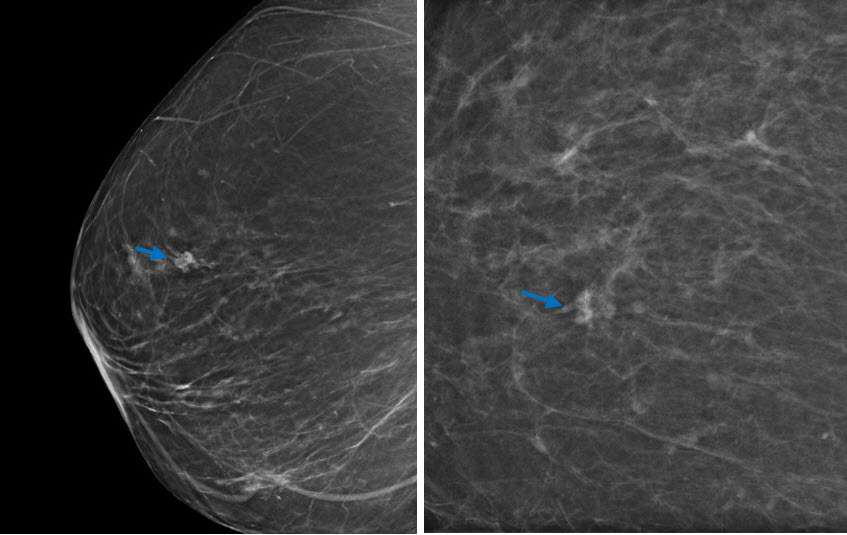
It is most rare for RSLs to present as grouped microcalcifications. The calcifications in these lesions demonstrate nonspecific morphology and are usually associated with coexisting proliferative changes and sclerosing adenosis within and surrounding the lesion(3).
Ultrasound:
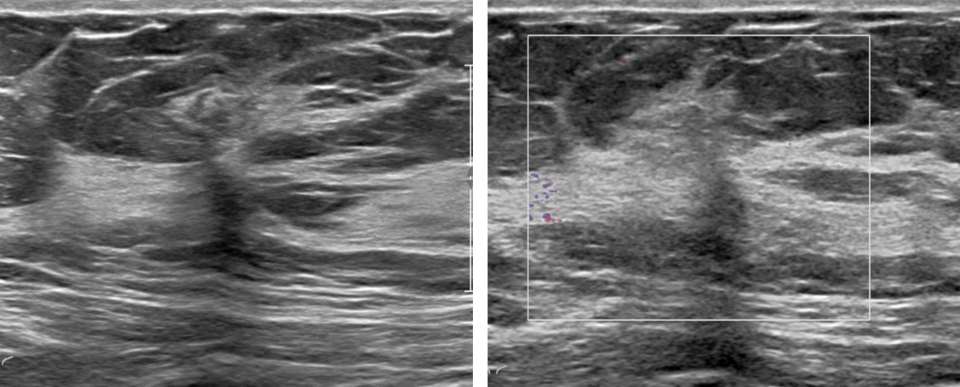
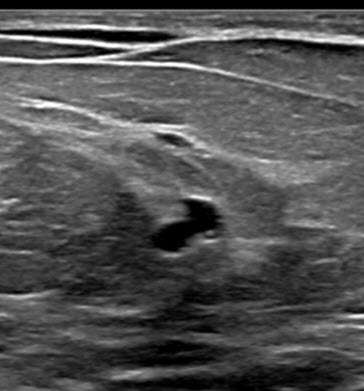
Radial sclerosing lesions have a variable appearance on ultrasound imaging and are frequently not visualized. They are typically found on targeted ultrasound examination for a mammographically detected lesion. When they are seen, they may present as an irregular hypoechoic mass or parenchymal distortion with ill-defined borders +/-posterior acoustic shadowing (Fig 1b). They may also present as a round or oval mass (Fig 2b) or a focal area of shadowing without a discrete mass. Findings that do not confirm but may favor a RSL over carcinoma include the absence of an echogenic halo, shadowing, or breast architectural distortion and/or the presence of tiny cystic changes(2,3).
MRI:
Radial sclerosing lesions may also be occult on MRI. When they are identified, they most commonly present as an irregular enhancing mass, which appears similar to carcinoma in morphology and enhancement characteristics (2). They may also present as an architectural distortion with no to mild enhancement, a benign appearing oval or round mass with mild and gradual enhancement, an enhancing focus, or non-mass enhancement (3)(Fig 3). While MRI examinations cannot reliably distinguish RSLs from carcinoma, these exams play an important role in excluding the presence of additional lesions in the affected or contralateral breast.
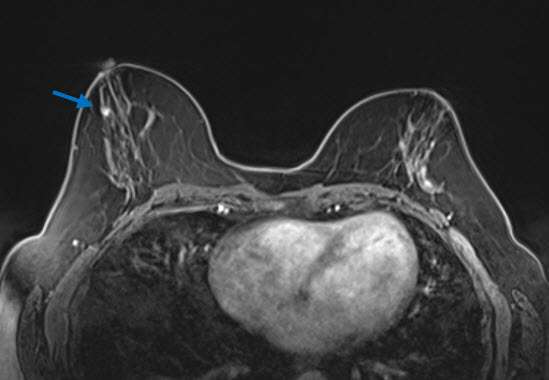
Management:
Management of radial sclerosing lesions is controversial. Surgical excision remains the traditional management practice. The upgrade rate of RSLs to carcinoma at surgical excision is widely variable throughout the literature. Studies show that upgrade rates for RSLs depends on the presence or absence of atypia at biopsy. RSLs without atypia have a reported upgrade rate ranging from 0-2.2%, while those with atypia range from 28-36%(4). There is also a higher risk of RSL upgrade in patients older than 50 years in age. Given the low upgrade rate of RSLs without atypia, there may be a role for imaging surveillance in these cases(2). There is currently no consensus on the specifics of imaging surveillance protocols or intervals. Ultimately, the management of complex sclerosing lesions is evolving via a multi-disciplinary approach and consideration of individual patient risk factors, lesion size, sampling technique and volume, histologic features, and radiologic-pathologic correlation.
References:
- Ha SM, Cha JH, Shin HJ, Chae EY, Choi WJ, Kim HH, Oh HY. "Radial Scars/complex Sclerosing Lesions of the Breast: Radiologic and Clinicopathologic Correlation." BMC Med Imaging. 2018 Nov 3;18(1):39. . PMID: 30390667; PMCID: PMC6215659.
- Manzar BZ, Phillips J, Dibble EH, Quintana LM, Lourenco AP. "Imaging and Management of Radial Scars and Complex Sclerosing Lesions." Radiographics. 2023 Oct;43(10):e230022. . PMID: 37733620.
- Trombadori CML, D'Angelo A, Ferrara F, Santoro A, Belli P, Manfredi R. "Radial Scar: A Management Dilemma." Radiol Med. 2021 Jun;126(6):774-785. . Epub 2021 Mar 20. PMID: 33743143; PMCID: PMC8154762.
- Yan P, DeMello L, Baird GL, Lourenco AP. "Malignancy Upgrade Rates of Radial Sclerosing Lesions at Breast Cancer Screening." Radiol Imaging Cancer. 2021 Nov;3(6):e210036. . PMID: 34766844; PMCID: PMC8637224.